PbS sensitized TiO2 nanotube arrays with different sizes and filling degrees for enhancing photoelectrochemical properties†
Abstract
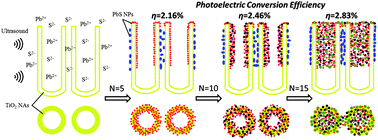
PbS nanoparticles (PbS NPs), an efficient sensitizer for TiO2 nanotube arrays (TiO2 NAs), were fabricated by the method of sonication-assisted successive ionic layer adsorption and reaction (SILAR). The filling degree and size of PbS NPs can be tuned by changing the repeated cycles (N) of the SILAR process. TiO2 NAs can be fully covered with PbS NPs with a size ranging from less than 4 nm to 25 nm and large aggregates inside and outside the nanotubes when N reaches 15. The growth mechanism of PbS NPs in TiO2 NAs was expounded in great detail in this work. Ultraviolet-visible diffuse-reflectance spectra and surface photovoltage spectroscopy were used to investigate the light absorption properties and the transfer behavior of photogenerated charges in PbS-modified TiO2 NA heterostructures. Results show that the absorption range of TiO2 NAs is extended from the ultraviolet to the visible region by PbS NPs modification. A heterojunction is formed between PbS NPs and TiO2 NAs, facilitating the separation of photogenerated charge carriers. This PbS NPs fully-covered TiO2 NA electrode exhibits the best photoelectrochemical performance in all PbS-sensitized TiO2 NA electrodes, due to a larger number of small PbS NPs (<4 nm). With AM 1.5G illumination at 100 mW cm−2, its short-circuit current density, open-circuit voltage and photoelectric conversion efficiency are 9.55 mA cm−2, 0.95 V and 2.83%, respectively.


 Please wait while we load your content...
Please wait while we load your content...