Inhibited phase behavior of gas hydrates in graphene oxide: influences of surface and geometric constraints†
Abstract
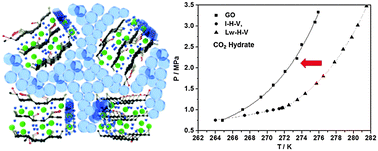
Porous materials have provided us unprecedented opportunities to develop emerging technologies such as molecular storage systems and separation mechanisms. Pores have also been used as supports to contain gas hydrates for the application in gas treatments. Necessarily, an exact understanding of the properties of gas hydrates in confining pores is important. Here, we investigated the formation of CO2, CH4 and N2 hydrates in non-interlamellar voids in graphene oxide (GO), and their thermodynamic behaviors. For that, low temperature XRD and P–T traces were conducted to analyze the water structure and confirm hydrate formation, respectively, in GO after its exposure to gaseous molecules. Confinement and strong interaction of water with the hydrophilic surface of graphene oxide reduce water activity, which leads to the inhibited phase behavior of gas hydrates.

 Please wait while we load your content...
Please wait while we load your content...