Resonant orbitals in fluorinated epitaxial graphene
Abstract
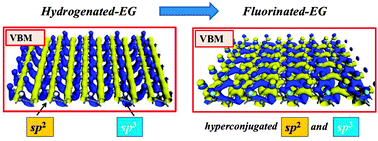
Fluorinated epitaxial graphene has potential applications in organic electronics. We present the calculation results by means of first-principles density-functional-theory for various fluorination patterns. Our results indicate that semi-fluorinated graphene conformations follow the same energetic order as the corresponding hydrogenated graphene counterparts. The distinctive electronic properties between semi-hydrogenated graphene and semi-fluorinated graphene are attributed to the polar covalent C–F bond in contrast to the covalent C–H bond. The partial ionic character of the C–F bond results in the hyperconjugation of C–F σ-bonds with an sp2 network of graphene. Resonant orbitals stabilize the stirrup conformation via the gauche effect. Resonant orbitals also lead to electron doping of the sp2 network and enhanced excitonic effect. The implications of resonant-orbital-induced doping for the electronic and magnetic properties of fluorinated epitaxial graphene are discussed.

 Please wait while we load your content...
Please wait while we load your content...