The radiosensitivity of 5- and 6-bromocytidine derivatives – electron induced DNA degradation
Abstract
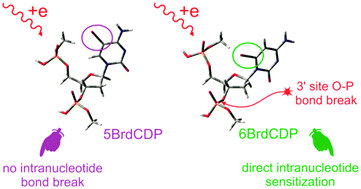
Halogenated nucleotides belong to the group of radiosensitizers that sensitize solid tumors when incorporated into genomic DNA. Here, we consider the propensity of two isomeric bromocytidine derivatives, 3′,5′-diphosphates of 5-bromo-2′-deoxycytidine (5BrdCDP) and 6-bromo-2′-deoxycytidine (6BrdCDP), to be damaged by electrons – one of the most abundant products formed during radiotherapy. An intranucleotide degradation mechanism leading to phosphodiester bond breakage (a model of single strand breakage in labeled DNA) and a ketone derivative formation was found for 6BrdCDP, while for 5BrdCDP a similar mechanism is sterically hindered. 5BrdCDP is, therefore, suggested to undergo electron induced degradation involving hydrogen transfer from a neighboring nucleotide or environment.

 Please wait while we load your content...
Please wait while we load your content...