Atomic charge transfer-counter polarization effects determine infrared CH intensities of hydrocarbons: a quantum theory of atoms in molecules model†
Abstract
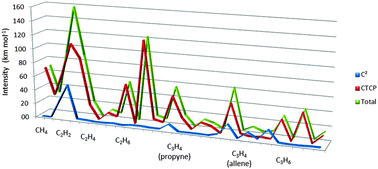
Atomic charge transfer-counter polarization effects determine most of the infrared fundamental CH intensities of simple hydrocarbons, methane, ethylene, ethane, propyne, cyclopropane and allene. The quantum theory of atoms in molecules/charge–charge flux–dipole flux model predicted the values of 30 CH intensities ranging from 0 to 123 km mol−1 with a root mean square (rms) error of only 4.2 km mol−1 without including a specific equilibrium atomic charge term. Sums of the contributions from terms involving charge flux and/or dipole flux averaged 20.3 km mol−1, about ten times larger than the average charge contribution of 2.0 km mol−1. The only notable exceptions are the CH stretching and bending intensities of acetylene and two of the propyne vibrations for hydrogens bound to sp hybridized carbon atoms. Calculations were carried out at four quantum levels, MP2/6-311++G(3d,3p), MP2/cc-pVTZ, QCISD/6-311++G(3d,3p) and QCISD/cc-pVTZ. The results calculated at the QCISD level are the most accurate among the four with root mean square errors of 4.7 and 5.0 km mol−1 for the 6-311++G(3d,3p) and cc-pVTZ basis sets. These values are close to the estimated aggregate experimental error of the hydrocarbon intensities, 4.0 km mol−1. The atomic charge transfer-counter polarization effect is much larger than the charge effect for the results of all four quantum levels. Charge transfer-counter polarization effects are expected to also be important in vibrations of more polar molecules for which equilibrium charge contributions can be large.

 Please wait while we load your content...
Please wait while we load your content...