β-Phenyl quenching of 9-phenylphenalenones: a novel photocyclisation reaction with biological implications†
Abstract
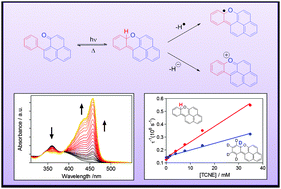
The singlet and triplet excited states of 9-phenylphenalenones 1 undergo β-phenyl quenching (BPQ) via addition of the carbonyl oxygen to the ortho position of the phenyl substituent. This reaction leads to the formation of naphthoxanthenes 4, which, in the absence of quenchers, undergo a very rapid electrocyclic ring opening reaction reverting to 1 within a few microseconds. Naphthoxanthene 4a contains a remarkably weak C–H bond, which enables efficient hydrogen transfer reactions to suitable acceptors, giving rise to the production of the naphthoxanthenyl radical or the naphthoxanthenium cation, depending on the solvent polarity. The study uncovers a number of new aspects of BPQ and suggests an excited state-mediated metabolic pathway in the biosynthesis of plant fluorones.


 Please wait while we load your content...
Please wait while we load your content...