Decomposition of nitrous oxide on Fe-doped boron nitride nanotubes: the ligand effect†
Abstract
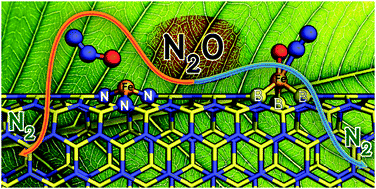
N2O decomposition on iron–doped boron nitride nanotubes (Fe–BNNTs) was investigated by means of the density functional theory (M06-L). Two different forms of Fe–BNNTs, which are substitutions of the Fe atom into the boron-vacancy and nitrogen-vacancy sites of BNNTs, were used as the catalyst. Influence of the support plays a crucial role in the electronic configuration and catalytic reactivity of the iron atom. With the nitrogen surrounding (Fe(B)–BNNT), the iron behaves as a Lewis acid for accepting an electron from the lone-pair orbital of the N2O oxygen atom (η1-O complex). The catalytic process over this one at the transition state involves a synergistic σ-donation from the HOMO of N2O into a LUMO of the catalyst and the π-back-bonding from the metal d orbital into the π* orbital of N2O, leading to the cleavage of the N–O bond. The activation for this step is 22.5 kcal mol−1. With the boron surrounding (Fe(N)–BNNT), the iron acting as a Lewis base plays a different role as compared with the iron in the case of Fe(B)–BNNTs. The HOMO of Fe(N)–BNNTs promotes the side-on binding mode of N2O on the iron center (η2-O,N complex), leading to the weakening of the N–O bond at the adsorption state. As a result, the decomposition over the Fe(N)–BNNTs takes place easily without an energy barrier.

 Please wait while we load your content...
Please wait while we load your content...