Diffusive and non-diffusive photo-induced proton coupled electron transfer from hydrogen bonded phenols to meso-tetrakis-5,10,15,20-pentafluorophenyl porphyrin†
Abstract
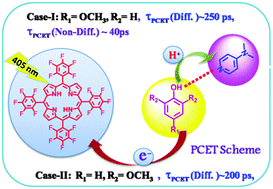
Enhanced reductive fluorescence quenching of meso-tetrakis-5,10,15,20-pentafluorophenyl porphyrin (H2F20TPP) by two different phenols, 4-methoxy phenol (4-MeOPhOH) and 2,6-dimethoxy phenol (2,6-DiMeOPhOH) in the presence of various pyridine bases in dichloromethane solution is studied using steady state and time resolved fluorescence spectroscopic methods by employing time correlated single photon counting (TCSPC) and fluorescence up-conversion techniques. An enhanced quenching behaviour of H2F20TPP is observed when phenols are hydrogen bonded to various pyridine bases. Quenching observed in the steady state and time resolved studies in the nanosecond time domain follows second order kinetics and generates quenching rate constants and hydrogen bond equilibrium constants, the latter of which agree quite closely with those obtained from independent spectroscopic measurements. A significant kinetic deuterium isotope effect is observed, indicating the importance of proton movement in the quenching processes. This quenching effect is attributed to be due to a tri-molecular transition state involving H2F20TPP and a hydrogen bonded phenol complex, in which electron transfer from phenol to excited H2F20TPP is concerted with proton movement from the phenol to hydrogen bonded base. Observed quenching behaviours are rationalized by invoking diffusion controlled proton coupled electron transfer. Fluorescence up-conversion studies in the 100 ps time domain confirm ultrafast PCET for 4-MeOPhOH and base pairs which fall in a non-diffusive regime.

 Please wait while we load your content...
Please wait while we load your content...