The complex, non-monotonic thermal response of the volumetric space of simple liquids†
Abstract
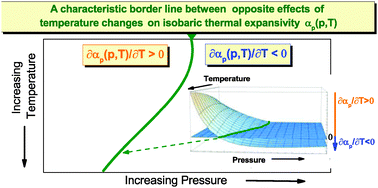
In this paper, an intricate effect of the isothermal compression of simple liquids on their volumetric response is reported for α,ω-halogenoalkanes as examples. We apply an accurate experimental technique, scanning transitiometry, to directly measure isobaric thermal volume expansivities αp of the liquids in a wide density range. To thoroughly analyze the observed intersection of the experimental isothermal pressure dependences of αp, we develop a class of equations of state derived in the density scaling regime for molecular dynamics, finding successful temperature parameterizations of an isothermal equation of state (EOS) intrinsically adapted to describe volumetric data in an extremely wide density range. The EOS based analyses of the scanning transitiometry data as a function of temperature T and pressure p undoubtedly show that the previously considered crossing point of the isothermal dependences αp(p) is in general represented by a non-linear and non-monotonic curve in the (T–p) phase diagram.

 Please wait while we load your content...
Please wait while we load your content...