Effects of temperature and ammonia flow rate on the chemical vapour deposition growth of nitrogen-doped graphene†
Abstract
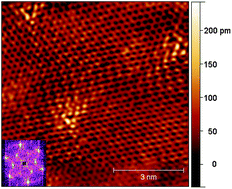
We doped graphene in situ during synthesis from methane and ammonia on copper in a low-pressure chemical vapour deposition system, and investigated the effect of the synthesis temperature and ammonia concentration on the growth. Raman and X-ray photoelectron spectroscopy was used to investigate the quality and nitrogen content of the graphene and demonstrated that decreasing the synthesis temperature and increasing the ammonia flow rate results in an increase in the concentration of nitrogen dopants up to ca. 2.1% overall. However, concurrent scanning electron microscopy studies demonstrate that decreasing both the growth temperature from 1000 to 900 °C and increasing the N/C precursor ratio from 1/50 to 1/10 significantly decreased the growth rate by a factor of six overall. Using scanning tunnelling microscopy we show that the nitrogen was incorporated mainly in substitutional configuration, while current imaging tunnelling spectroscopy showed that the effect of the nitrogen on the density of states was visible only over a few atom distances.

 Please wait while we load your content...
Please wait while we load your content...