Halogen-abstraction reactions from chloromethane and bromomethane molecules by alkaline-earth monocations
Abstract
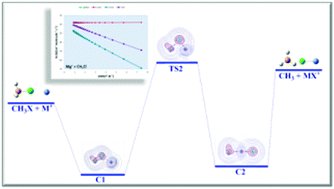
The reactions, in the gas phase, between alkali-earth monocations (Mg+, Ca+, Sr+, Ba+) and CH3X (X = Cl, Br) have been theoretically studied. The stationary points on the potential energy surfaces were characterized at the Density Functional Theory level on the framework of the mPW1K functional with the QZVPP Ahlrichs's basis sets. A complementary kinetics study has also been performed using conventional/variational microcanonical transition state theory. In the reactions of Mg+ with either chloro- or bromomethane the transition structure lies in energy clearly above the reactants rendering thermal activation of CH3Cl or CH3Br extremely improbable. The remaining reactions are exothermic and barrierless processes; thus carbon–halogen bonds in chloro- or bromomethane can be activated by calcium, strontium or barium monocations to obtain the metal halogen cation and the methyl radical. The Mulliken population analysis for the stationary points of the potential energy surfaces supports a “harpoon”-like mechanism for the halogen-atom abstraction processes. An analysis of the bonding situation for the stationary points on the potential energy surface has also been performed in the framework of the quantum theory of atoms in molecules.

 Please wait while we load your content...
Please wait while we load your content...