Adsorption and photocatalytic splitting of water on graphitic carbon nitride: a combined first principles and semiempirical study
Abstract
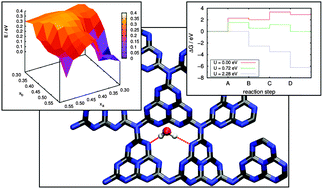
Graphitic carbon nitride, g-C3N4, is a promising organic photo-catalyst for a variety of redox reactions. In order to improve its efficiency in a systematic manner, however, a fundamental understanding of the microscopic interaction between catalyst, reactants and products is crucial. Here we present a systematic study of water adsorption on g-C3N4 by means of density functional theory and the density functional based tight-binding method as a prerequisite for understanding photocatalytic water splitting. We then analyze this prototypical redox reaction on the basis of a thermodynamic model providing an estimate of the overpotential for both water oxidation and H+ reduction. While the latter is found to occur readily upon irradiation with visible light, we derive a prohibitive overpotential of 1.56 eV for the water oxidation half reaction, comparing well with the experimental finding that in contrast to H2 production O2 evolution is only possible in the presence of oxidation cocatalysts.

 Please wait while we load your content...
Please wait while we load your content...