Electronically tunable anion−π interactions in pyrylium complexes: experimental and theoretical studies†
Abstract
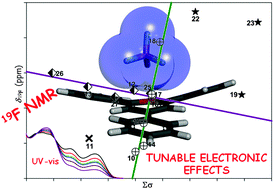
Noncovalent interactions of anions with electron-deficient aromatic rings that have been studied so far involve non-heteroaromatic or nitrogen-based heteroaromatic systems. Here we report the first case of an organic oxygenated aromatic system, in particular the tri-aryl-pyrylium tetrafluoroborate system, for which noncovalent anion–π interactions of the pyrylium cation with the tetrafluoroborate anion have been experimentally detected and demonstrated by means of 19F NMR spectroscopy in solution. A series of pyrylium tetrafluoroborate salts were synthesized in the presence of BF3·Et2O, by direct reaction of 4-substituted benzaldehydes with 4-substituted acetophenones or via the previously obtained chalcone of the less reactive ketone. Correlations of 19F NMR chemical shifts of tetrafluoroborate anion for most of the synthesized tri-arylpyrylium tetrafluoroborate complexes with both the pyrylium cation molecular weight and the standard substituent Hammett constants, demonstrate anion–π+ interaction to act between the polyatomic anion BF4− and the pyrylium aromatic system. DFT calculations reveal that an additional (C–H)+–anion hydrogen bond involving the H(5) of pyrylium ring exists for these fluorescent dyes that show a tunable cup-to-cap shape cavity. The strong fluorescence emission observed for some representative pyrylium tetrafluoroborates described herein, makes them a promising class of tunable emission wavelength dyes for laser technology applications.

 Please wait while we load your content...
Please wait while we load your content...