Computational study of structural properties of lithium cation complexes with carbamate-modified disiloxanes†
Abstract
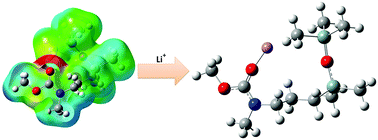
Lithium cation solvation structures [Li(S)n=1–4]+ with ligands of cyclic or noncyclic carbamate-modified disiloxanes are optimized at B3LYP level of theory and compared to their corresponding simplified carbamates and to the organic carbonates ethylene carbonate (EC) and dimethyl carbonate (DMC). The electrostatic potentials (ESP) of these investigated carbonyl-containing solvents are mapped on the electron density surface. The maximum ESP is located at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O-oxygen, whereas the disiloxane functionality represents an unpolar residue. Natural Bond Orbitals (NBO) analysis reveals strong n(N) → π(C
O-oxygen, whereas the disiloxane functionality represents an unpolar residue. Natural Bond Orbitals (NBO) analysis reveals strong n(N) → π(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) donor–acceptor interactions in carbamates which outrun dipolar properties. As a result, higher total binding energies (ΔEB) for solvation of Li+ in carbamates (−148 kcal mol−1) are found than for carbonates (−137 kcal mol−1). Furthermore, the disiloxane moiety with its Si–O bond is stabilized by n(O) → σ*(Si–C) hyperconjugation that provides additional electron density to a nearby SiCH3 methyl group thus supporting an additional SiCH2–H⋯Li+ coordination. The formation of all investigated solvation structures is exothermic. Owing to steric hindrance of noncyclic carbonyl-containing ligands and the bulky disiloxane functionality, the solvation structure [Li(S)3]+ is the preferred structure according to Gibbs free energy ΔGB results.
O) donor–acceptor interactions in carbamates which outrun dipolar properties. As a result, higher total binding energies (ΔEB) for solvation of Li+ in carbamates (−148 kcal mol−1) are found than for carbonates (−137 kcal mol−1). Furthermore, the disiloxane moiety with its Si–O bond is stabilized by n(O) → σ*(Si–C) hyperconjugation that provides additional electron density to a nearby SiCH3 methyl group thus supporting an additional SiCH2–H⋯Li+ coordination. The formation of all investigated solvation structures is exothermic. Owing to steric hindrance of noncyclic carbonyl-containing ligands and the bulky disiloxane functionality, the solvation structure [Li(S)3]+ is the preferred structure according to Gibbs free energy ΔGB results.

 Please wait while we load your content...
Please wait while we load your content...