Anomalous high adsorption energy of H2O on fluorinated graphenes: a first principles study†
Abstract
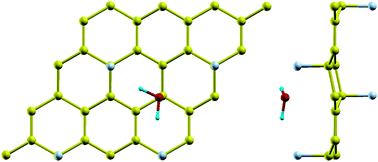
Polytetrafluoroethylene (PTFE) has been well-known for the surface superhydrophobicity, while its two-dimensional stable analogues, CF and C4F, possess distinct surface wettability. The CF inherits the hydrophobicity from PTFE since the van der Waals interaction is mitigated by the high electronegativity of fluorine. Surprisingly, a high adsorption energy (∼550 meV per molecule) of water has been unveiled on C4F via density functional theory studies, implying anomalous superhydrophilicity of C4F. The abrupt transition from hydrophobicity of CF to superhydrophilicity of C4F can be reconciled with the difference in their molecular orbitals. The high adsorption energy of C4F is mainly attributed to the Coulomb attraction among the non-bonding interactions, as proposed by our theoretical model. Since the surface chemical inertness of CF inhibits it from being widely adopted in device fabrication, the present finding suggests that C4F can be a promising candidate in graphene-based electronic devices.

 Please wait while we load your content...
Please wait while we load your content...