Sol-flame synthesis of cobalt-doped TiO2 nanowires with enhanced electrocatalytic activity for oxygen evolution reaction†
Abstract
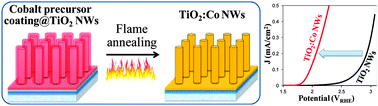
Doping nanowires (NWs) is of crucial importance for a range of applications due to the unique properties arising from both impurities' incorporation and nanoscale dimensions. However, existing doping methods face the challenge of simultaneous control over the morphology, crystallinity, dopant distribution and concentration at the nanometer scale. Here, we present a controllable and reliable method, which combines versatile solution phase chemistry and rapid flame annealing process (sol-flame), to dope TiO2 NWs with cobalt (Co). The sol-flame doping method not only preserves the morphology and crystallinity of the TiO2 NWs, but also allows fine control over the Co dopant profile by varying the concentration of Co precursor solution. Characterizations of the TiO2:Co NWs show that Co dopants exhibit 2+ oxidation state and substitutionally occupy Ti sites in the TiO2 lattice. The Co dopant concentration significantly affects the oxygen evolution reaction (OER) activity of TiO2:Co NWs, and the TiO2:Co NWs with 12 at% of Co on the surface show the highest OER activity with a 0.76 V reduction of the overpotential with respect to undoped TiO2 NWs. This enhancement of OER activity for TiO2:Co NWs is attributed to both improved surface charge transfer kinetics and increased bulk conductivity.

 Please wait while we load your content...
Please wait while we load your content...