Linear and nonlinear optical properties of V-shaped D–π–A–π–D chromophores: effects of the incorporation of aromatic rings in the polyenic π-bridges of open-chain ketocyanines†‡
Abstract
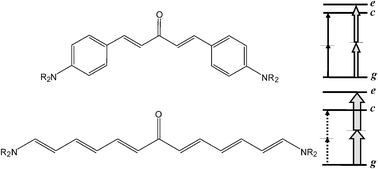
Following previous studies on α and β polarizabilities of ketocyanines, a subgroup of D–π–A–π–D quadrupolar chromophores with moderately V-shaped structure, the present work analyses the effects of modifying the π-bridges connecting the D (NMe2) and A (CO) groups. This aim is pursued through a detailed comparison between the previously studied ketocyanines (KC2, KC3) and a Michler's ketone analogue (KM1) bearing styrenic (in the place of polyenic) π-bridges. First, we report a spectroscopic study, including absorption and fluorescence anisotropy spectra, aimed to probe the electronic peculiarities of KM1 as well as to derive consistent three-state model (TSM) parameters for the three compounds. The paper goes on with an extensive theoretical study, carried out in the framework of the density functional theory (DFT), encompassing the structure, the electronic spectrum, α and β polarizabilities and two-photon absorption (TPA) cross-sections (σTP). Calculations performed according to the sum-over-states (SOS) approach are discussed with reference to the performances of few-state descriptions, it is shown that such descriptions (including TSM), which have been proved to be quite reliable in the case of KC2 and KC3, lose their effectiveness with KM1 because of the electronic characteristics related to the styrenic π-bridges. As to the TPA cross-sections, the results of TSM and SOS approaches concerning the TSM g → c and g → e transitions are supplemented by those obtained using the quadratic response theory. A common qualitative conclusion, traceable to the degree of bending of the V-shaped structure, is that in the case of KM1 the allowed (g → e) and the “forbidden” (g → c) transitions both should be observable in the TPA spectrum, as confirmed by experiment.

 Please wait while we load your content...
Please wait while we load your content...