IRMPD spectroscopy of metalated flavins: structure and bonding of Mq+–lumichrome complexes (Mq+ = Li+–Cs+, Ag+, Mg2+)†
Abstract
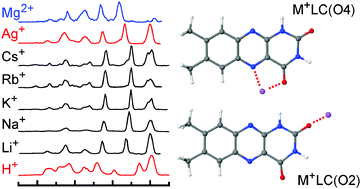
Infrared multiphoton dissociation (IRMPD) spectra of mass selected isolated metal–lumichrome ionic complexes, Mq+LCn with Mq+ = Li+, Na+, K+, Rb+, Cs+, Ag+ (n = 1), and Mg2+ (n = 2), are recorded in the fingerprint range. The complexes are generated in an electrospray ionization source coupled to an ion cyclotron mass spectrometer and the IR free electron laser FELIX. Vibrational and isomer assignments of the IRMPD spectra are accomplished by density functional theory calculations at the B3LYP/cc-pVDZ level, which provide insight into the structure, binding energy, bonding mechanism, and spectral properties of the complexes. The two major binding sites identified involve metal bonding to the oxygen atoms of the two available carbonyl groups of LC (denoted O2 and O4). The more stable O4 isomer benefits from an additional interaction with the lone pair of the nearby N5 atom of LC. While M+LC with alkali metals are mainly stabilized by electrostatic forces, the Ag+LC complex reveals additional stabilization arising from partly covalent contributions. Finally, the interaction of Mg2+ ions with LC is largely enhanced by the doubled positive charge. The frequencies of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes are a sensitive indicator of both the metal binding site and the metal bond strength.
O stretching modes are a sensitive indicator of both the metal binding site and the metal bond strength.

 Please wait while we load your content...
Please wait while we load your content...