A reduced radial potential energy function for the halogen bond and the hydrogen bond in complexes B⋯XY and B⋯HX, where X and Y are halogen atoms
Abstract
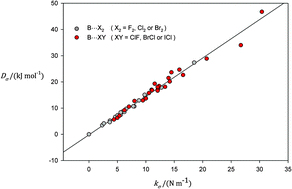
It is shown by considering 76 halogen- and hydrogen-bonded complexes B⋯XY and B⋯HX (where B is a Lewis base N2, CO, C2H2, C2H4, H2S, HCN, H2O, PH3 or NH3 and X, Y are F, Cl, Br or I) that the intermolecular stretching force constants kσ (determined from experimental centrifugal distortion constants via a simple model) and the intermolecular dissociation energies Dσ (calculated at the CCSD(T)(F12*)/cc-pVDZ-F12 level of theory) are related by Dσ = Cσkσ, where Cσ = 1.50(3) × 103 m2 mol−1. This suggests that one-dimensional functions implying direct proportionality of Dσ and kσ, (e.g. a Morse or Rydberg function) might serve as reduced radial potential energy functions for such complexes.

 Please wait while we load your content...
Please wait while we load your content...