Hydrogen-bonded intermediates and transition states during spontaneous and acid-catalyzed hydrolysis of the carcinogen (+)-anti-BPDE†
Abstract
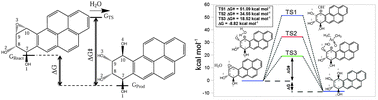
Understanding mechanisms of (+)-anti-BPDE detoxification is crucial for combating its mutagenic and potent carcinogenic action. However, energetic-structural correlations of reaction intermediates and transition states during detoxification via hydrolysis are poorly understood. To gain mechanistic insight we have computationally characterized intermediate and transition species associated with spontaneous and general-acid catalyzed hydrolysis of (+)-anti-BPDE. We studied the role of cacodylic acid as a proton donor in the rate limiting step. The computed activation energy (ΔG‡) is in agreement with the experimental value for hydrolysis in a sodium cacodylate buffer. Both types of, spontaneous and acid catalyzed, BPDE hydrolysis can proceed through low-entropy hydrogen bonded intermediates prior to formation of transition states whose energies determine reaction activation barriers and rates.

 Please wait while we load your content...
Please wait while we load your content...