Tunable electronic and magnetic properties in germanene by alkali, alkaline-earth, group III and 3d transition metal atom adsorption
Abstract
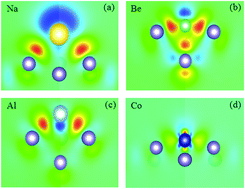
We performed first-principles calculations to study the adsorption characteristics of alkali, alkali-earth, group III, and 3d transition-metal (TM) adatoms on germanene. We find that the adsorption of alkali or alkali-earth adatoms on germanene has minimal effects on geometry of germanene. The significant charge transfer from alkali adatoms to germanene leads to metallization of germanene, whereas alkali-earth adatom adsorption, whose interaction is a mixture of ionic and covalent, results in semiconducting behavior with an energy gap of 17–29 meV. For group III adatoms, they also bind germanene with mixed covalent and ionic bonding character. Adsorption characteristics of the transition metals (TMs) are rather complicated, though all TM adsorptions on germanene exhibit strong covalent bonding with germanene. The main contributions to the strong bonding are from the hybridization between the TM 3d and Ge pz orbitals. Depending on the induced-TM type, the adsorbed systems can exhibit metallic, half-metallic, or semiconducting behavior. Also, the variation trends of the dipole moment and work function with the adsorption energy across the different adatoms are discussed. These findings may provide a potential avenue to design new germanene-based devices in nanoelectronics.

 Please wait while we load your content...
Please wait while we load your content...