Controlled growth of conical nickel oxide nanocrystals and their high performance gas sensing devices for ammonia molecule detection†
Abstract
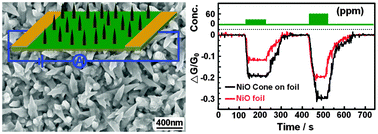
NiO nanocones with good symmetry and highly ordered structure on NiO foil substrate have been successfully fabricated via a facile wet chemical approach combined with subsequent high temperature oxidation. These organized conical superstructures grow only along a certain direction and be controlled via the self-assembly and oriented attachment of a nucleus, which mainly rely on the similar surface energies and the extent of lattice matching of the oriented attached surfaces. During high temperature oxidation, the electric field created via the Ni2+ and O2− facilitates Ni2+ diffusion outward along the grain boundaries and O2− diffusion inward toward to meet the Ni2+ ions, forming NiO. The as-grown NiO nanocones are 50–350 nm in diameter and 50–400 nm in height. The tip diameter of the nanocone is about 30 nm and the apex angle of the nanocone is about 40°. Meanwhile, we systematically investigated the gas sensing properties of the sensors based on the as-fabricated NiO foil covered with nanocone arrays for ammonia detection at room temperature. The results show that the gas sensing devices have outstanding sensitivity, reproducibility and selectivity, which are mainly because of the excellent connection between the NiO sensing materials and the Au electrodes, the strong electron donating ability of ammonia and the large active surface of selective physisorption for ammonia.

 Please wait while we load your content...
Please wait while we load your content...