Bonding and spectroscopic properties of complexes of SO2–O2 and SO2–N2 and its atmospheric consequences
Abstract
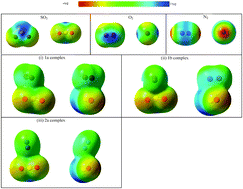
van der Waals complexes of sulfur dioxide (SO2) with oxygen (O2) and nitrogen (N2) have been investigated by using MP2 and aug-cc-pVXZ (X = D, T) basis set. Two minimum structures with symmetry C1 and Cs have been located at the intermolecular potential energy surface (IPES) of the complex of SO2–O2. Stacked Cs structure of SO2–O2 is found to have greater stability than C1 structure. In the case of SO2–N2, one minimum structure with Cs symmetry has been characterized. In this study, CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ and interaction energy calculation at complete basis set (CBS) limit has been employed for better energetic description. The natural bond orbital (NBO) calculation demonstrates the bonding in terms of charge transfer from X-atom lone pair of X2 (X = O or N) to the antibonding SO orbital of SO2. The strength of various intra and inter bonds in the complexes were calculated in terms of electron density at bond critical points (BCP) using quantum theory of atoms in molecules (QTAIM). Frequency calculations for these complexes show a number of interactions induced by low frequency modes in the far IR region. Symmetry adapted calculation were also computed for the complexes and is established that the ratio of dispersion to induction effect is large for the most stable conformers. The atmospheric implications are also discussed for these complexes.

 Please wait while we load your content...
Please wait while we load your content...