Sputtering graphite coating to improve the elevated-temperature cycling ability of the LiMn2O4 electrode†
Abstract
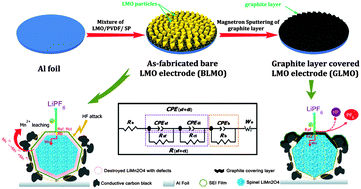
To improve the cycle performance of LiMn2O4 at elevated temperature, a graphite layer is introduced to directly cover the surface of a commercial LiMn2O4-based electrode via room-temperature DC magnetron sputtering. The as-modified cathodes display improved capacity retention as compared to the bare LiMn2O4 cathode (BLMO) at 55 °C. When sputtering graphite for 30 min, the sample shows the best cycling performance at 55 °C, maintaining 96.2% capacity retention after 200 cycles. Reasons with respect to the graphite layer for improving the elevated-temperature performance of LiMn2O4 are systematically investigated via the methods of cyclic voltammetry, electrochemical impedance spectroscopy, X-ray photoelectron spectrometry, scanning and transmission electron microscopy, X-ray diffraction and inductively coupled plasma-atomic emission spectrometry. The results demonstrate that the graphite coated LiMn2O4 cathode has much less increased electrode polarization and electrochemical impedance than BLMO during the elevated-temperature cycling process. Furthermore, the graphite layer is able to alleviate the severe dissolution of manganese ions into the electrolyte and mitigate the morphological and structural degradation of LiMn2O4 during cycling. A model for the electrochemical kinetics process is also suggested for explaining the roles of the graphite layer in suppressing the Mn dissolution.

 Please wait while we load your content...
Please wait while we load your content...