Theoretical simulation of reduction mechanism of graphene oxide in sodium hydroxide solution
Abstract
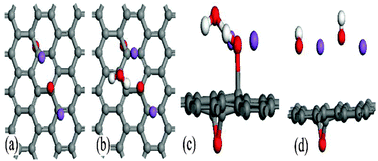
Based on a density functional theory simulation, we proposed a reduction mechanism of graphene oxide (GO) under a sodium hydroxide solution containing anions (OH−), cations (Na+) and neutral H2O molecules as main components. OH− anion can interact with hydroxyl in GO and transfer electrons to the graphene sheet, resulting in negatively charged GO, and these electrons obviously lower the barrier of the ring-opening reaction of epoxy. Na+ cations can be attracted by the negatively charged GO, and this reaction is equivalent to the one between metallic Na and GO. The opened epoxy is reduced with the assistance of Na+ cation and water molecule. In such a reduction process, NaOH can be viewed as a catalyst and more defects should be formed because of these diffused epoxies on the negatively charged graphene sheet. Our results may be helpful to understand further the nature of the reduction of GO among various reducing agents.

 Please wait while we load your content...
Please wait while we load your content...