What are preferred water–aromatic interactions in proteins and crystal structures of small molecules?†
Abstract
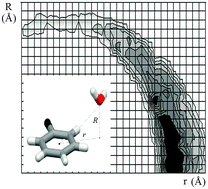
The distribution of water molecules around aromatic rings in the protein structures and crystal structures of small molecules shows quite a small number of the strongest OH–π interactions, a larger number of parallel interactions, and the largest number of the weakest CH–O interactions.

 Please wait while we load your content...
Please wait while we load your content...