Competition between weak hydrogen bonds: C–H⋯Cl is preferred to C–H⋯F in CH2ClF–H2CO, as revealed by rotational spectroscopy†
Abstract
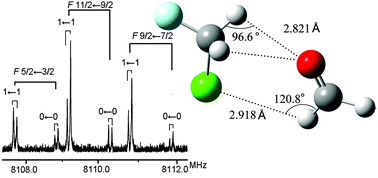
We recorded the pulsed jet Fourier transform microwave spectrum of the 1 : 1 adduct of CH2ClF with formaldehyde. Formaldehyde is linked to CH2ClF through a C–H⋯Cl bond rather than a weak C–H⋯F hydrogen bond, with a H⋯Cl “bond length” of 2.918 Å. Two additional equivalent C–H⋯O contacts, with a H⋯O distance of 2.821 Å, characterize the complex. Tunnelling splittings due to the internal rotation of the formaldehyde moiety have been observed, which allowed estimating the barrier to the internal rotation of formaldehyde to be 125(10) cm−1. The 35Cl quadrupole coupling constants have been determined to be χaa = 31.131(7) MHz and χbb–χcc = −105.82(1) MHz.

 Please wait while we load your content...
Please wait while we load your content...