Observation of highly decoupled conductivity in protic ionic conductors
Abstract
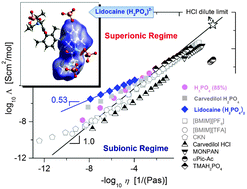
Ionic liquids (ILs) are key materials for the development of a wide range of emerging technologies. Protic ionic liquids, an important class of ILs, have long been envisioned as promising anhydrous electrolytes for fuel cells. It is well known that in comparison to all other cations, protons exhibit abnormally high conductivity in water. Such superprotonic dynamics was expected in protic ionic conductors as well. However, many years of extensive studies led to the disappointing conclusion that this is not the case and most protic ionic liquids display subionic behavior. Therefore, the relatively low conductivity seems to be the main obstacle for the application of protic ionic liquids in fuel cells. Using dielectric spectroscopy, herein we report the observation of highly decoupled conductivity in a newly synthesized protic ionic conductor. We show that its proton transport is strongly decoupled from the structural relaxation, in terms of both temperature dependence and characteristic rates. This finding offers a fresh look on the charge transport mechanism in PILs and also provides new ideas for design of anhydrous materials with exceptionally high proton conductivity.

 Please wait while we load your content...
Please wait while we load your content...