The origin of high activity but low CO2 selectivity on binary PtSn in the direct ethanol fuel cell†
Abstract
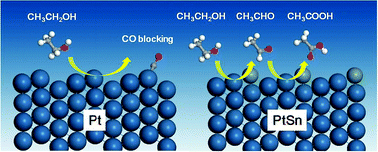
The most active binary PtSn catalyst for direct ethanol fuel cell applications has been studied at 20 °C and 60 °C, using variable temperature electrochemical in situ FTIR. In comparison with Pt, binary PtSn inhibits ethanol dissociation to CO(a), but promotes partial oxidation to acetaldehyde and acetic acid. Increasing the temperature from 20 °C to 60 °C facilitates both ethanol dissociation to CO(a) and then further oxidation to CO2, leading to an increased selectivity towards CO2; however, acetaldehyde and acetic acid are still the main products. Potential-dependent phase diagrams for surface oxidants of OH(a) formation on Pt(111), Pt(211) and Sn modified Pt(111) and Pt(211) surfaces have been determined using density functional theory (DFT) calculations. It is shown that Sn promotes the formation of OH(a) with a lower onset potential on the Pt(111) surface, whereas an increase in the onset potential is found upon modification of the (211) surface. In addition, Sn inhibits the Pt(211) step edge with respect to ethanol C–C bond breaking compared with that found on the pure Pt, which reduces the formation of CO(a). Sn was also found to facilitate ethanol dehydrogenation and partial oxidation to acetaldehyde and acetic acid which, combined with the more facile OH(a) formation on the Pt(111) surface, gives us a clear understanding of the experimentally determined results. This combined electrochemical in situ FTIR and DFT study provides, for the first time, an insight into the long-term puzzling features of the high activity but low CO2 production found on binary PtSn ethanol fuel cell catalysts.

 Please wait while we load your content...
Please wait while we load your content...