Ab initio calculations on the 1O2 quenching mechanism by trans-resveratrol†
Abstract
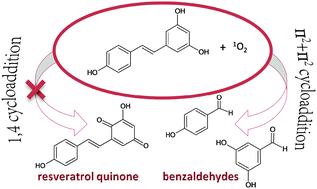
trans-Resveratrol has shown to play an important role in a variety of biological and medical processes such as reactive oxygen species (ROS) scavenging, inhibition of apoptosis and induction of cell survival. In light of the fact that resveratrol and its oligomers were found to be selective singlet oxygen 1O2 quenchers, we report here a systematic study on the reactivity of trans-resveratrol toward molecular oxygen in acetone simulated media. On the basis of the controversial hypotheses reported in the literature we explored, at density functional levels of theory, two different mechanisms. The first one leads to a resveratrol quinone product via an endoperoxide intermediate by attack of 1O2 on the resorcinol ring, assisted (pathway (b)) or not (pathway (a)) by a water molecule. The second mechanism, in which the singlet oxygen reacts with the double bond connecting the two resveratrol rings leading to benzaldehyde products, involves the formation of a dioxetane intermediate. As the outcomes of our computational analysis show that the latter mechanism is kinetically more favorable than the former one, it is likely that when trans-resveratrol reacts with singlet oxygen a dioxetane intermediate is formed.

 Please wait while we load your content...
Please wait while we load your content...