Roles of water molecules in trapping carbon dioxide molecules inside the interlayer space of graphene oxides†
Abstract
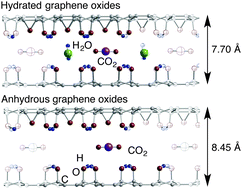
Density functional theory (DFT) calculations were employed to investigate the energetics of carbon dioxide migration within hydrated or anhydrous graphene oxides (GOs). When anhydrous GO structures contain a carbon dioxide molecule, the carbon dioxide interacts repulsively with the GO layers to increase the interlayer spacing. The repulsive electrostatic interactions are reduced by the insertion of water molecules into CO2-containing GO structures due to the occurrence of attractive water–layer interactions through hydrogen bonding. Consequently, the interlayer spacings in CO2-containing hydrated structures are shortened compared with those in the anhydrous structures. The results indicate that the intercalated water molecules have the ability to connect the GO layers in the presence of carbon dioxide. Furthermore, the DFT calculations indicated that the GO interlayer spacings, which are influenced by the intercalation of water molecules, control carbon dioxide migration within the GO layers. The importance of the interlayer spacings on the migration of carbon dioxide arises from the occurrence of repulsive interactions between CO2 and oxygen-containing groups attached on the graphene sheets. When the GO interlayer spacings are short due to the presence of intercalated water molecules, the repulsive interactions between carbon dioxide and the GO layers are strong enough to prevent CO2 from migrating from its original position. Such repulsive interactions do not occur during the migration of CO2 within anhydrous GO structures because of the relatively longer interlayer spacing. Accordingly, CO2 migrates within anhydrous GO with a less significant barrier, indicating that carbon dioxide molecules are easily released from the GO.

 Please wait while we load your content...
Please wait while we load your content...