Electrodeposition of iron and iron–aluminium alloys in an ionic liquid and their magnetic properties
Abstract
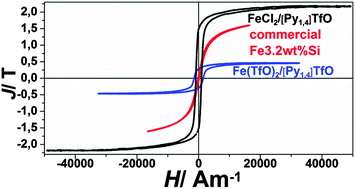
In this work we show that nanocrystalline iron and iron–aluminium alloys can be electrodeposited from the ionic liquid 1-butyl-1-methylpyrrolidinium trifluoromethylsulfonate, [Py1,4]TfO, at 100 °C. The study comprises CV, SEM, XRD, and magnetic measurements. Two different sources of iron(II) species, Fe(TfO)2 and FeCl2, were used for the electrodeposition of iron in [Py1,4]TfO. Cyclic voltammetry was employed to evaluate the electrochemical behavior of FeCl2, Fe(TfO)2, and (FeCl2 + AlCl3) in the employed ionic liquid. Thick iron deposits were obtained from FeCl2/[Py1,4]TfO at 100 °C. Electrodeposition of iron–aluminium alloys was successful in the same ionic liquid at 100 °C. The morphology and crystallinity of the obtained deposits were investigated using SEM and XRD, respectively. XRD measurements reveal the formation of iron–aluminium alloys. First magnetic measurements of some deposits gave relatively high coercive forces and power losses in comparison to commercial iron–silicon samples due to the small grain size in the nanometer regime. The present study shows the feasibility of preparing magnetic alloys from ionic liquids.

 Please wait while we load your content...
Please wait while we load your content...