The molecular configuration of a DOPA/ST monolayer at the air–water interface: a molecular dynamics study†
Abstract
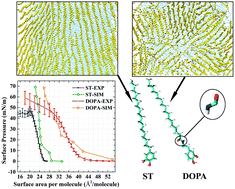
In this study, surface pressure–area isotherms for N-stearoyldopamine (DOPA) and 4-stearylcatechol (ST) monolayers are obtained by means of molecular dynamics simulations and compared to experimental isotherms. The difference between DOPA and ST is an amide group, which is present in the alkyl tails of DOPA molecules. We find a large difference between the isotherms for DOPA and ST monolayers. Upon using TIP4P/2005 for water and OPLS force fields for the organic material and a relatively large system size, the simulated results are found to be consistent with experiments. With molecular dynamics simulations, the configurations of molecules in the monolayers can be directly analyzed. When the surface pressure is high, a regular molecular orientation is observed for ST molecules, whereas regular orientations are only observed in local domains for DOPA molecules. The differences between DOPA and ST monolayers are attributed to the amide groups in DOPA molecules, which are useful for both steric effects and the formation of hydrogen bonds in the DOPA monolayers. This study clearly demonstrates that hydrogen bonds, due to the presence of the amide group in DOPA, are the cause of the disorder in its Langmuir monolayers. Thus, the conclusion may be helpful in making ordered organic monolayers in the future.

 Please wait while we load your content...
Please wait while we load your content...