Synthesis of poly(ethylene furandicarboxylate) polyester using monomers derived from renewable resources: thermal behavior comparison with PET and PEN†
Abstract
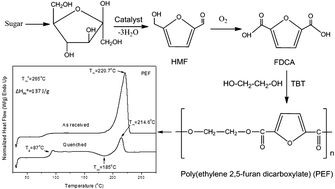
Poly(ethylene-2,5-furandicarboxylate) (PEF) is a new alipharomatic polyester that can be prepared from monomers derived from renewable resources like furfural and hydroxymethylfurfural. For this reason it has gained high interest recently. In the present work it was synthesized from the dimethylester of 2,5-furandicarboxylic acid and ethylene glycol by applying the two-stage melt polycondensation method. The thermal behavior of PEF was studied in comparison to its terephthalate and naphthalate homologues poly(ethylene terephthalate) (PET) and poly(ethylene naphthalate) (PEN), which were also synthesized following the same procedure. The equilibrium melting point of PEF was found to be 265 °C while the heat of fusion for the pure crystalline PEF was estimated to be about 137 J g−1. The crystallization kinetics was analyzed using various models. PET showed faster crystallization rates than PEN and this in turn showed faster crystallization than PEF, under both isothermal and non-isothermal conditions. The spherulitic morphology of PEF during isothermal crystallization was investigated by polarized light microscopy (PLM). A large nucleation density and a small spherulite size were observed for PEF even at low supercoolings, in contrast to PET or PEN. Thermogravimetric analysis indicated that PEF is thermally stable up to 325 °C and the temperature for the maximum degradation rate was 438 °C. These values were a little lower than those for PET or PEN.

 Please wait while we load your content...
Please wait while we load your content...