Correlated fluorine diffusion and ionic conduction in the nanocrystalline F− solid electrolyte Ba0.6La0.4F2.4—19F T1(ρ) NMR relaxation vs. conductivity measurements
Abstract
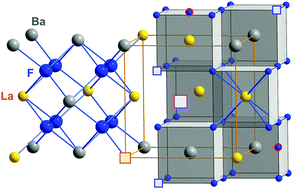
Chemical reactions induced by mechanical treatment may give access to new compounds whose properties are governed by chemical metastability, defects introduced and the size effects present. Their interplay may lead to nanocrystalline ceramics with enhanced transport properties being useful to act as solid electrolytes. Here, the introduction of large amounts of La into the cubic structure of BaF2 served as such an example. The ion transport properties in terms of dc-conductivity values of the F− anion conductor Ba1−xLaxF2+x (here with x = 0.4) considerably exceed those of pure, nanocrystalline BaF2. So far, there is only little knowledge about activation energies and jump rates of the elementary hopping processes. Here, we took advantage of both impedance spectroscopy and 19F NMR relaxometry to get to the bottom of ion jump diffusion proceeding on short-range and long-range length scales in Ba0.6La0.4F2.4. While macroscopic transport is governed by an activation energy of 0.55 to 0.59 eV, the elementary steps of hopping seen by NMR are characterised by much smaller activation energies. Fortunately, we were able to deduce an F− self-diffusion coefficient by the application of spin-locking NMR relaxometry.

 Please wait while we load your content...
Please wait while we load your content...