Spectroscopic investigation of interaction between bovine serum albumin and amine-functionalized silicon quantum dots†
Abstract
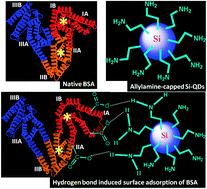
We have investigated the dynamics and mechanistic details of the interaction between bovine serum albumin (BSA) and allylamine-capped silicon quantum dots (Si QDs) by means of fluorescence spectroscopy, circular dichroism (CD), and FTIR spectroscopy. The intrinsic fluorescence of BSA gets quenched in the presence of Si QDs due to ground-state complex formation. The binding stoichiometry and various thermodynamic parameters have been evaluated by using the van't Hoff equation. It has been observed that the association process is driven by a favourable negative enthalpy change with an unfavorable negative entropy change. These results have been explained by considering specific hydrogen bonding interactions between amine moieties (–NH2) of Si QDs and carboxylate groups (–COO−) of aspartate (Asp) and glutamate (Glu) residues of BSA. Circular dichroism (CD) and FTIR spectroscopy revealed nominal changes in the secondary structure of the adsorbed proteins due to partial unfolding of the native protein upon surface adsorption while the overall tertiary structure remains close to that of the native state.

 Please wait while we load your content...
Please wait while we load your content...