Controllable synthesis, morphology evolution and electrochemical properties of LiFePO4 cathode materials for Li-ion batteries
Abstract
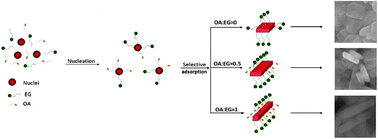
Monodispersed LiFePO4 nanocrystals with diverse morphologies were successfully synthesized via a mild and controllable solvothermal approach with a mixture of ethylene glycol and oleic acid as the solvent. Morphology evolution of LiFePO4 nanoparticles from nanoplates to nanorods can be simply realized by varying the volume ratio of oleic acid to ethylene glycol. Moreover, the mechanism of competitive adsorption between ethylene glycol and oleic acid was proposed for the formation of different morphologies. Electrochemical measurements show that the LiFePO4/C nanorods have an initial discharge capacity of 155 mA h g−1 at 0.5 C with a capacity retention of 80% at a high rate of 5 C, which confirms that LiFePO4/C nanorods exhibit excellent rate capability and cycling stability.

 Please wait while we load your content...
Please wait while we load your content...