Confined H2O molecules as local probes of pressure-induced amorphisation in faujasite
Abstract
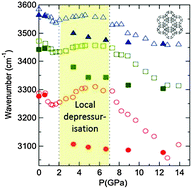
Confined H2O molecules act as local probes for depressurization phenomena during the pressure induced amorphisation of faujasite NaX at which the OH stretching frequency first decreases and then increases almost to its room pressure value upon further compression. Pair distribution function (PDF) analysis provides evidence that amorphisation corresponds to a collapse of the structure around hydrated sodium cations with strong distortion of the secondary building units (double six-membered rings, sodalite cages). Both the use of guest molecules as local probes in far- and mid-infrared spectroscopy, where we correlate intermolecular water H bonding vibrations and internal mode behaviour under confinement, and PDF analysis could be of great use to study the mechanical behaviour of other hydrated materials.

 Please wait while we load your content...
Please wait while we load your content...