A new consumable anode material of titanium oxycarbonitride for the USTB titanium process
Abstract
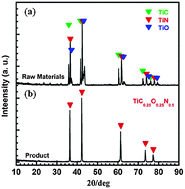
A single phase titanium oxycarbonitride TiC0.25O0.25N0.5 was prepared by sintering a homogenous mixture of TiO, TiC and TiN with a molar ratio of 1 : 1 : 2 by spark plasma sintering (SPS) at 1873 K. TiO0.25C0.25N0.5 was then used as the consumable anode for the USTB titanium process and the anode dissolution process was investigated by electrochemical methods. The results showed that TiO0.25C0.25N0.5 was electrochemically dissolved into Ti2+ in the NaCl–KCl melts as determined by square-wave voltammetry analysis and simultaneously CO as well as N2 evolved in the anode as detected by mass spectroscopy. And TiO0.25C0.25N0.5 has exhibited a similar electron transfer resistance as TiC0.5O0.5 and TiN as measured by electrochemical impedance spectroscopy (EIS) analysis. By galvanostatic electrolysis, the cathode products were proved to be pure titanium powders. The results indicated that TiO0.25C0.25N0.5 is a suitable consumable anode for the USTB titanium process.

 Please wait while we load your content...
Please wait while we load your content...