Enriching physisorption of H2S and NH3 gases on a graphane sheet by doping with Li adatoms
Abstract
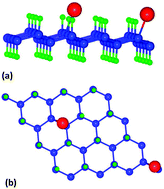
We have used density functional theory to investigate the adsorption efficiency of a hydrogenated graphene (graphane) sheet for H2S and NH3 gases. We find that neither the pristine graphane sheet nor the sheet defected by removing a few surface H atoms have sufficient affinity for either H2S or NH3 gas molecules. However, a graphane sheet doped with Li adatoms shows a strong sensing affinity for both the mentioned gas molecules. We have calculated the absorption energies with one [referred to as half coverage] molecule and two molecules [referred to as full coverage] for both gases with the Li-doped graphane sheet. We find that for both the gases, the calculated absorption energies are adequate enough to decide that the Li-doped graphane sheet is suitable for sensing H2S and NH3 gases. The Li-doped sheet shows a higher affinity for the NH3 gas compared to the H2S gas molecules due to a stronger Li(s)–N(p) hybridization compared to that of Li(s)–S(p). However, while going from the half coverage effect to the full coverage effect, the calculated binding energies show a decreasing trend for both the gases. The calculated work function of the Li-doped graphane sheet decreases while bringing the gas molecules within its vicinity, which explains the affinity of the sheet towards both the gas molecules.

 Please wait while we load your content...
Please wait while we load your content...