On the use of thioamides as fluorescence quenching probes for tracking protein folding and stability
Abstract
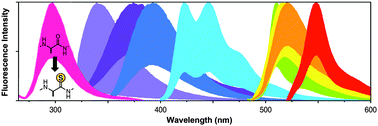
Our laboratory has developed thioamide analogs of the natural amino acids as minimally-perturbing fluorescence quenching probes that can be placed at many locations in a protein sequence. We have shown that the mechanism of quenching can be either Förster resonance energy transfer (FRET) or photoinduced electron transfer (PET), depending on the identity of the donor fluorophore. Furthermore, we have shown that one can use a combination of semi-synthetic methods to label full-sized proteins with fluorophore–thioamide pairs. These probes can be used to study protein–protein interactions, protein folding or misfolding, and proteolysis.
 Please wait while we load your content...
Please wait while we load your content...