Low-temperature combustion chemistry of novel biofuels: resonance-stabilized QOOH in the oxidation of diethyl ketone†
Abstract
The Cl˙ initiated oxidation reactions of diethyl ketone (DEK; 3-pentanone; (CH3CH2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2,2,4,4-d4-diethyl ketone (d4-DEK; (CH3CD2)2C
O), 2,2,4,4-d4-diethyl ketone (d4-DEK; (CH3CD2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1,1,1,5,5,5-d6-diethyl ketone (d6-DEK; (CD3CH2)2C
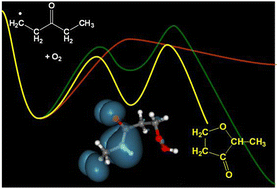
O) and 1,1,1,5,5,5-d6-diethyl ketone (d6-DEK; (CD3CH2)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are studied at 8 Torr and 550–650 K using Cl2 as a source for the pulsed-photolytic generation of Cl˙. Products are monitored as a function of reaction time, mass, and photoionization energy using multiplexed photoionization mass spectrometry with tunable synchrotron radiation. Adding a large excess of O2 to the reacting flow allows determination of products resulting from oxidation of the initial primary (Rp) and secondary (Rs) radicals formed via the Cl˙ + DEK reaction. Because of resonance stabilization, the secondary DEK radical (3-oxopentan-2-yl) reaction with O2 has a shallow alkyl peroxy radical (RsO2) well and no energetically low-lying product channels. This leads to preferential back dissociation of RsO2 and a greater likelihood of consumption of Rs by competing radical–radical reactions. On the other hand, reaction of the primary DEK radical (3-oxopentan-1-yl) with O2 has several accessible bimolecular product channels. Vinyl ethyl ketone is observed from HO2-elimination from the DEK alkylperoxy radicals, and small-molecule products are identified from β-scission reactions and decomposition reactions of oxy radical secondary products. Although channels yielding OH + 3-, 4-, 5- and 6-membered ring cyclic ether products are possible in the oxidation of DEK, at the conditions of this study (8 Torr, 550–650 K) only the 5-membered ring, 2-methyltetrahydrofuran-3-one, is observed in significant quantities. Computation of relevant stationary points on the potential energy surfaces for the reactions of Rp and Rs with O2 indicates this cyclic ether is formed via a resonance-stabilized hydroperoxyalkyl radical (QOOH) intermediate, formed from isomerization of the RpO2 radical.
O) are studied at 8 Torr and 550–650 K using Cl2 as a source for the pulsed-photolytic generation of Cl˙. Products are monitored as a function of reaction time, mass, and photoionization energy using multiplexed photoionization mass spectrometry with tunable synchrotron radiation. Adding a large excess of O2 to the reacting flow allows determination of products resulting from oxidation of the initial primary (Rp) and secondary (Rs) radicals formed via the Cl˙ + DEK reaction. Because of resonance stabilization, the secondary DEK radical (3-oxopentan-2-yl) reaction with O2 has a shallow alkyl peroxy radical (RsO2) well and no energetically low-lying product channels. This leads to preferential back dissociation of RsO2 and a greater likelihood of consumption of Rs by competing radical–radical reactions. On the other hand, reaction of the primary DEK radical (3-oxopentan-1-yl) with O2 has several accessible bimolecular product channels. Vinyl ethyl ketone is observed from HO2-elimination from the DEK alkylperoxy radicals, and small-molecule products are identified from β-scission reactions and decomposition reactions of oxy radical secondary products. Although channels yielding OH + 3-, 4-, 5- and 6-membered ring cyclic ether products are possible in the oxidation of DEK, at the conditions of this study (8 Torr, 550–650 K) only the 5-membered ring, 2-methyltetrahydrofuran-3-one, is observed in significant quantities. Computation of relevant stationary points on the potential energy surfaces for the reactions of Rp and Rs with O2 indicates this cyclic ether is formed via a resonance-stabilized hydroperoxyalkyl radical (QOOH) intermediate, formed from isomerization of the RpO2 radical.

 Please wait while we load your content...
Please wait while we load your content...