The role of site-directed point mutations in protein misfolding†
Abstract
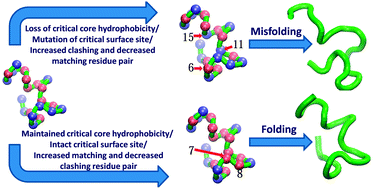
A self-consistent mean-field based model is presented to explore the role of site-directed point mutations in designing folded and/or misfolded sequences with a reduced hydrophobic–polar (HP) patterning of amino acids. This site-directed point mutation procedure is developed and applied to both real and lattice proteins to generate a diverse set of sequences. The respective roles of core and surface residues are analyzed with respect to the optimum hydrophobicity required for the structural stability of the protein. The core sites are found to have a critical number of hydrophobic residues, below which a protein may misfold, while the surface sites show a clear preference for the polar residues with the ability to tolerate some hydrophobic residues. Although core sites play an important role in the structural stability of proteins, some specific surface sites are also found to be equally important. A clash and match calculation procedure is proposed, which may be used to predict the number of residue pairs in a sequence with unfavorable and favorable interactions, respectively, due to site-directed point mutations. The number of clashing and matching residue pairs may indicate whether the mutated sequence would be folded or misfolded. The results are independent of the secondary structure topology of the protein. This model may provide new insights into the effect of point mutations on protein stability and may introduce a new method to predict the outcome of a mutation in terms of its probability to fold or misfold.

 Please wait while we load your content...
Please wait while we load your content...