Solvent induced enhancement of enantiomeric excess: a case study of the Henry reaction with cinchona thiourea as the catalyst†
Abstract
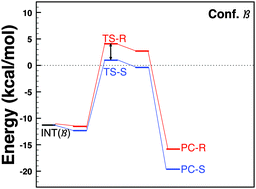
Enantiomeric excess (ee) in asymmetric catalysis may be strongly dependent on the solvent. The reaction product may range from an almost racemic mixture to an ee of over 90% for different solvents. We study this phenomenon for the C–C coupling reaction between nitromethane and benzaldehyde (the Henry reaction) with cinchona thiourea as the catalyst, where solvents that are strong Lewis bases induce a high ee. We show that the effect of the solvent does not consist of a change in the reaction mechanism. Instead, the solvation “prepares” the molecule, which is very flexible, in a specific conformation. The reaction barriers in this conformer are not lower than for other conformers, but are sufficiently differentiated between the enantiomers to give rise to a large ee. It is the strong Lewis basicity of the solvent that leads to the clear preference in solution for the “asymmetric” conformer. Although general rules or predictions for how solvent effects could be harnessed to produce a desired ee in general would be hard to formulate, this study does show that it is in this case (and presumably in many other cases as well) specific solute–solvent interactions rather than effects of the dielectric continuum of the solvent that are the root cause of the solvent effect. This is in agreement with experiment for the Henry reaction.

 Please wait while we load your content...
Please wait while we load your content...