Understanding the effect of vibrational excitation in reaction dynamics: the Ne + H2+(v = 0–17, j = 1) → NeH+ + H, Ne + H+ + H proton transfer and dissociation cross sections†
Abstract
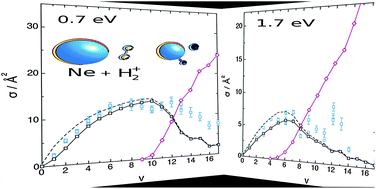
The dependence of the cross section (σ) of the Ne + H2+ → NeH+ + H proton transfer reaction on the vibrational excitation of H2+, v = 0–17 and j = 1, was analyzed in detail at the collision energies (Ecol) of 0.7 and 1.7 eV, using the quasi-classical trajectory (QCT) method and the PHHJ3 and LZHH potential energy surfaces (PESs), taking advantage of the rich experimental data available for this reaction as a function of H2+(v). The efficiency of vibrational excitation to promote the reaction was investigated from the analysis of the σ(QCT) vs. v dependence in terms of the average reaction probability, maximum impact parameter, regions of the (late barrier) PES explored, and taking into account the Ne + H2+ → Ne + H+ + H dissociative channel, which plays a dominant role at high enough total energies. Although the earlier PHHJ3 PES performs rather well, the LZHH PES QCT results show a better agreement with the experiment. On the other hand, some artifacts were found in recently reported QCT calculations (unphysical oscillations in σ(QCT) as a function of v), and the present study shows that special care is needed when carrying out QCT calculations involving highly excited vibrational states.

 Please wait while we load your content...
Please wait while we load your content...