The non-covalent nature of the molecular structure of the benzene molecule†
Abstract
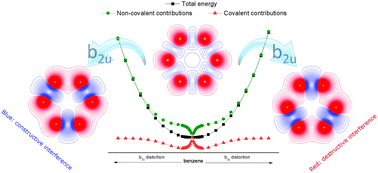
The benzene molecule is one of the most emblematic systems in chemistry, with its structural features being present in numerous different compounds. We have carried out an analysis of the influence of quantum mechanical interference on the geometric features of the benzene molecule, showing that many of the characteristics of its equilibrium geometry are a consequence of non-covalent contributions to the energy. This result implies that quasi-classical reasoning should be sufficient to predict the defining aspects of the benzene structure such as its planarity and equivalence of its bond lengths.

 Please wait while we load your content...
Please wait while we load your content...