Abstract
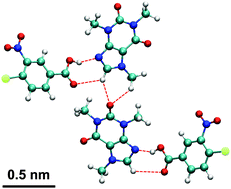
Complementing recent experimental results, here we report a computational study of remarkably flexible, elastically bendable caffeine cocrystals (cocrystal solvate 1), formed from caffeine (CAF), 4-chloro-3-nitrobenzoic acid (CNB), and methanol, and compare with its unsolvated brittle form, 1 (dry). We show that 1 is able to maintain stable cocrystal structures at temperatures between 100 K and 400 K. The tensile and compressive Young's modulus of 1 are close to ∼10 GPa. The ultimate strength is more than 600 MPa in tensile and 400 MPa in compressive at temperature of 100 K. The simulation results of the structural and mechanical properties of 1 are in good agreement with our previous experimental work. Notably, before the ultimate tensile stress, the stress-to-strain curves of 1 show linear behavior, but 1 (dry) show nonlinear behavior. This study might explain the remarkable elasticity of 1 and is relevant to the design of high-performance organic materials with excellent self-healing or efficient stress dissipating properties.

 Please wait while we load your content...
Please wait while we load your content...