Study of water adsorption and capillary bridge formation for SiO2 nanoparticle layers by means of a combined in situ FT-IR reflection spectroscopy and QCM-D set-up†
Abstract
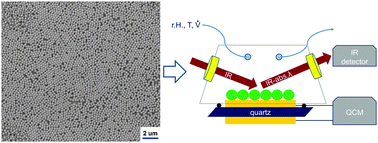
Water adsorption and capillary bridge formation within a layer of SiO2-nanoparticles were studied in situ by means of a combination of quartz crystal microbalance (QCM-D) with dissipation analysis and Fourier transformation infrared reflection absorption spectroscopy (FT-IRRAS). FT-IR data were employed to distinguish the “ice-like” and “liquid-like” contributions and to support the analysis of the QCM-D data concerning mass change and dissipation. Combined measurements show that for SiO2-nanoparticles with a diameter of about 250 nm, the formation of two adsorbed monolayers of water as well as bulk water leads to a rather linear increase in the dissipation for relative humidity values of up to 60% which is followed by a strong increase in dissipation during the actual liquid bridge formation. Subsequently, the dissipation drops again when the relative humidity is further increased to values >90%.

 Please wait while we load your content...
Please wait while we load your content...