Elucidating the oxide growth mechanism on platinum at the cathode in PEM fuel cells†
Abstract
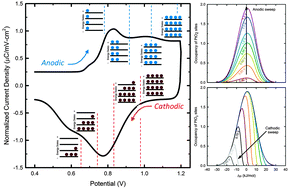
Simulations of platinum oxidation in literature have yet to fully replicate an experimental cyclic voltammogram. In this manuscript a mechanism for platinum oxidation is proposed based upon the results of in operando X-ray absorption spectroscopy, where it was found that PtO2 is present at longer hold times. A new method to quantify extended X-ray absorption fine structure data is presented, and the extent of oxidation is directly compared to electrochemical data. This comparison indicated that PtO2 was formed at the expense of an initial oxide species. From previous literature studies it can be concluded that the rate of platinum oxidation is not a function of only potential and coverage. To that end, the concept of a heterogeneous oxide layer was introduced into the model, whereby place-exchanged PtO2 structures of varying energy states are formed through a single transition state. This treatment allowed, for the first time, the simulation of the correct current–potential behavior at varying scan rates and upper potential limits.

 Please wait while we load your content...
Please wait while we load your content...