Photoluminescence quenching in compressed MgO nanoparticle systems†
Abstract
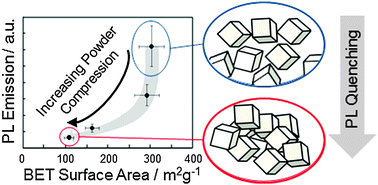
Efficient use of highly dispersed metal oxides for lighting, energy conversion and catalysis requires knowledge about the impact of density and microstructure of the powders on the optical nanoparticle properties. For MgO nanocube powders we present a combined photoluminescence (PL) and electron paramagnetic resonance (EPR) approach which enables for samples of different aggregation states the quantification of the fractional powder volume that becomes illuminated with UV and visible light during the PL measurements. Using O2 as a PL emission quencher and – after light induced exciton separation and oxygen adsorption – as an EPR active adsorbate we observed clear aggregation dependent trends in PL emission quenching that originate from particle–particle contacts. Upon interaction of low coordinated surface elements with the surfaces of adjacent MgO nanocubes, which occurs even at powder consolidation levels that escape sorption analysis, the radiative decay of excited surface states becomes quenched down to 15% of the original intensity. Our results underline the critical role of microstructure and the aggregation state of a nanoparticle ensemble with respect to spectroscopic properties and related adsorption induced changes.

 Please wait while we load your content...
Please wait while we load your content...